Customized manufacturing & assembly
solutions tailored to your needs.
Quality assurance measures ensuring consistent product excellence.

- Single-source partner – Beyond manufacturing solutions, we offer support that includes assembly, packaging, and sterilization management.
- Certified to deliver – As an ISO 13485:2016-certified, FDA-registered provider, we meet the rigorous standards of quality and compliance for medical device and life sciences industries.
- Automation expertise – We’re a leader in designing and building custom automation for dip molding, dip coating, and spray coating.
Medical device manufacturing & assembly solutions.
- High-volume precision molding & extrusion
- Sub-assemblies & finished devices
- Tip forming
- Microdrilling & punching
- Tube overmolding
- Skiving
- Tube flaring/cutting
- Coatings
- Part marking and decorating
- Welding & bonding
- Packaging
- Sterilization management
A range of environments to meet your project’s needs.
Streamlined manufacturing and assembly processes.
At Aptyx, we provide manufacturing and assembly services in a range of environments to meet the regulatory requirements of your medical component or device. The type of medical device you’re developing will determine which environment is right for your product. Our flexible manufacturing facilities allow us to scale up as needed to accommodate your product’s manufacturing and assembly requirements.
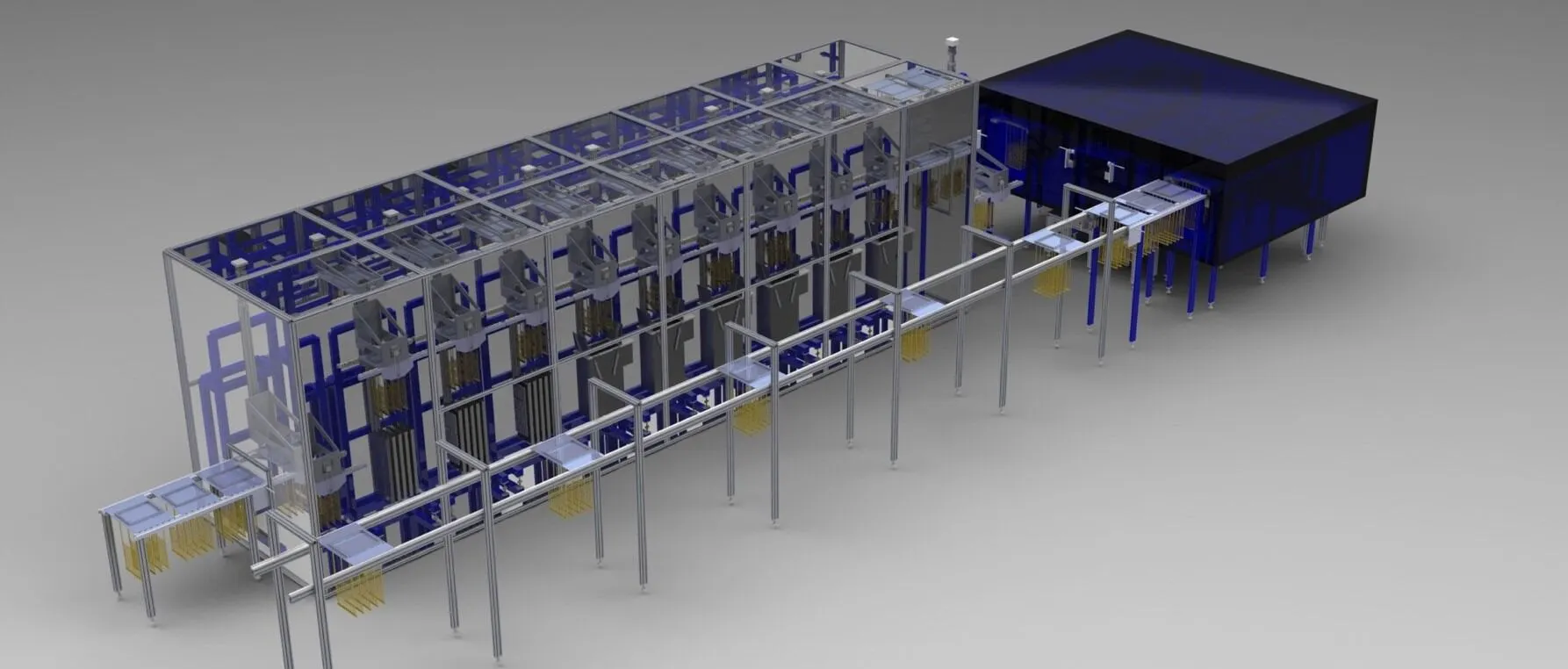
Custom equipment to meet your complex needs.
Aptyx is the leader in the design, manufacture, and installation of highly reliable and innovative dip molding and dip coating equipment for demanding applications including medical, pharmaceutical, electronics, industrial, automotive, and more. We provide end-to-end support for your coating project, from prototype lab to process development to equipment design and manufacture, including test and inspection services. In the R&D phase, our seasoned technical team can work with you to create a process development strategy by reviewing polymer options, tooling design, equipment requirements, and long-term production support needs.
Smart solutions
for complex markets.

Medical Device & Life Sciences

Industrial

Aerospace & Defense

Energy
Manufacturing & assembly resources
What cleanroom classification is required for medical device injection molding and assembly?
Read blog post
Startup leverages molding & complex assembly to deliver novel embolic catheter for 510(k) submission in only 6 months
Read study

