
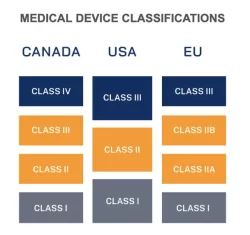
Figure 1
To properly manufacture and assemble medical-grade products, many companies seek medical device cleanrooms and white room manufacturing and assembly services. Aptyx helps medical device companies meet their specific development and manufacturing goals, offering ISO 7 & 8 cleanroom manufacturing and assembly services, along with white room and other environments. The type of medical device you require will determine which environment is right for your product. In the US medical devices are divided into Classes I-III with Class I posing the lowest risk to the patient. There are similar medical device classifications in the EU and Canada as illustrated in Figure 1.
While there is some overlap, it is generally safe to say that the higher the medical device class, the lower the cleanroom class. These and other factors will help match up your medical device type and class with the ideal manufacturing environment.
ISO 7 Cleanroom

Our highest level of controlled environment manufacturing and assembly services for medical devices, ISO 7 cleanrooms must maintain a filtration level of 10,000 particles per square foot of air. Strictly controlling the temperature, pressure, and humidity levels within this cleanroom environment ensures the air space is free of any contaminants, dust, or particles that could impact the functionality or cleanliness of the medical device. Some common examples of medical devices produced using ISO 7 cleanroom standards include stents, implants, catheters, medical balloons, and intravenous infusion pump systems.
ISO 8 Cleanroom
The next cleanroom we offer adheres to similar levels of cleanliness, maintaining a filtration level that assures no more than 100,000 particles per square foot of air. This cleanroom is commonly used to produce medical devices like catheters, syringes, surgical equipment, medical prep equipment, and various medical housings and casings for equipment. Having both ISO 7 and 8 cleanrooms, we can oftentimes manufacture, assemble, and package your medical devices in-house.
White Room Manufacturing
Nearly half of medical devices on the market fall under Class I – the least risk to the patient. Additionally, nearly all Class I devices do not need to meet strict regulatory requirements for cleanroom manufacturing. To help reduce cost, while still achieving an exceptionally clean production environment, we also offer white room manufacturing and assembly for medical devices like endotracheal tubes, gear assemblies for surgical devices and infusion pumps, and imaging equipment. These environments also help keep the production space clean through strict monitoring of air pressure, temperature, and humidity, without strict regulations of certified cleanrooms.
By offering a range of manufacturing and assembly environments, we help companies best match their product to the ideal environment. We also provide a full suite of services for complex components and devices, from molding and extrusion to coatings and assembly. Our team of experienced engineers and operators are committed to guiding your product to market success, with swift execution and results that endure. Reach out via our Contact Us page to see how we can help you with your project.

